Institute of Radiation Protection
The Institute of Radiation Protection is a specialised professional organisation with more than 30 years of experience in Slovakia. It focuses on radiation protection, the safe use of ionising radiation sources, and quality assurance in healthcare.
By combining technical expertise with a deep understanding of everyday healthcare operations, the Institute supports hospitals, outpatient clinics, and other operators in meeting regulatory requirements, improving transparency, and ensuring the safety of patients and staff.
Its services range from expert measurements and inspections to system-level solutions and digital tools that enable efficient, auditable, and sustainable radiation protection management.
What we do
Radiation protection in healthcare
Expert consulting, methodological guidance, and the design of protective measures for facilities using sources of ionising radiation.
Quality assurance
Testing and inspection of medical radiation equipment, verification of performance parameters and long-term stability, and support for the optimisation of clinical procedures.
Facility design and documentation
Preparation of technical documentation for the establishment, reconstruction, and operation of facilities, including layouts, technical designs, and process settings.
Personal dosimetry
Monitoring of occupational exposure, including the setup and operation of recording and control systems in compliance with radiation protection regulations.
Training and education
Professional training programmes, courses, and continuing education for staff working with ionising radiation, with practical support for implementation in daily practice.
Digital and data-driven solutions
Development and operation of digital systems for monitoring doses, quality, and documentation, supporting scalable, standardised, and auditable radiation protection processes.
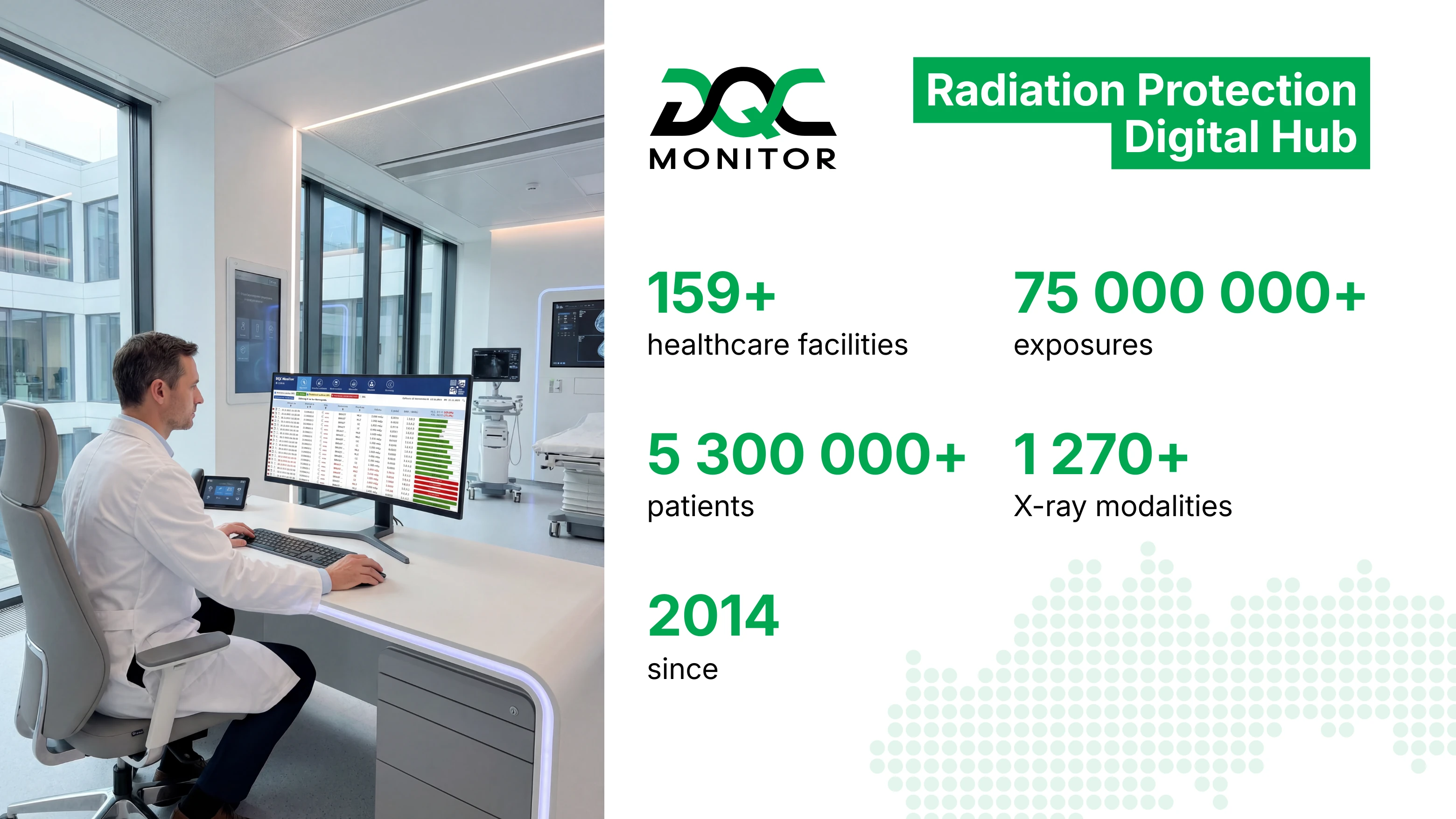
DQC monitor
DQC monitor is the Institute’s core system for medical radiation dose monitoring, developed and operated since 2014.
The system integrates data directly from diagnostic imaging modalities and healthcare information systems, enabling secure and standardised collection of medical exposure data across different healthcare facilities. Today, DQC monitor aggregates data from a wide network of public and private providers, creating a unified framework for monitoring exposures, quality, documentation, and reporting.
In an environment where fragmented data is a common challenge, DQC monitor provides clear visibility, comparability, and control.

Benefits for healthcare providers
-
Patient dose monitoring
Structured overviews of exposures by modality, facility, or examination type.
-
Optimisation support
Identification of trends, deviations, and opportunities to reduce unnecessary exposure while maintaining diagnostic quality.
-
Diagnostic Reference Levels (DRLs)
Comparison of doses with reference values to support internal quality management and external oversight.
-
Operational quality and safety
Centralised records of equipment tests, service events, control activities, and required documentation.
-
Statistics and reporting
Standardised outputs for management, professionals, and regulatory reporting.
-
Continuity of information across healthcare providers
Consolidation of data from multiple facilities, reducing duplication and improving clinical context for follow-up examinations.
Patient-oriented solutions
DQC monitor also serves as the technological foundation for future patient-oriented solutions, including the Radiation Exposure Passport for Patients.
Building on its established infrastructure, verified data flows, and existing integrations, the Institute is currently developing this concept as a patient-facing digital service. The aim is to enable patients, in a secure and controlled manner, to access a clear and reliable overview of their personal history of medical radiation exposure, while ensuring full compliance with data protection and regulatory requirements.